|
 |
The overall implantation procedure
for a neurostimulation system generally involves three
stages:
● Lead placement and intraoperative test stimulation
● Screening test period
● Complete system implantation with either the totally
implantable system or the radio frequency system (if the
screening test has been successful) |
 |
Preoperative
set up
Selecting the leads
Selecting the appropriate leads is essential for
successful neurostimulation. The process of lead
selection is based on patient information (chronology
and symptomatology of pain), the physicians diagnosis of
the patient’s pain type and pattern e.g. simple pain or
complex pain, and physician preference. Two basic types
of neurostimulation leads can be used with
neurostimulators:
● Percutaneous leads – Leads that are inserted through a
needle into the epidural space
● Surgical leads – leads that are implanted via a
laminectomy
Percutaneous leads have become increasingly popular
because the implantation procedure is substantially less
invasive. However, it is generally accepted that
surgical leads should be used when repeated migrations
occur or when the voltage required for paraesthesia is
high.
Selecting the implant site
The table below summarizes optimal lead sites by
vertebral body and pain location. It is important to
note that when the back is stimulated so are the legs.
For percutaneous leads the entry level is 1–2 vertebral
bodies below the target vertebra. For surgical leads,
the incision is usually made half a vertebrae below (or
at the bottom of) the target vertebra. |
| Table
1: Optimal lead sites by vertebrae and pain location |
| Pain location |
Lead tip level |
| Foot only |
T12–L1 |
| Lower leg and ankle |
T11–T12 |
| Knee and thigh |
T9–T10 |
| Thigh |
T9–T10 |
| Buttocks |
T9 |
| Lower back and lower limb |
T8–T9 |
| Upper chest wall/Precordium |
T1–T2 |
| Upper extremity/Upper Limb |
C3–C5 |
 |
Initial lead
placement
The protocol for initial lead placement is based on lead
type. A summary of two common techniques used for
percutaneous and surgical leads, respectively, is
highlighted below.
Percutaneous leads
Prior to beginning initial lead placement, the physician
uses fluoroscopy to mark the insertion or entry level
for the lead(s). Lead placement is performed under local
anesthesia within the sterile field. All the accessories
necessary for placement are provided in a lead kit. If
two leads are used, two lead kits are required. The
physician will begin the initial lead placement
procedure by:
● Identifying the vertebral body using fluoroscopy and
administering an anesthetic along the incision line
(Figure 1)
|
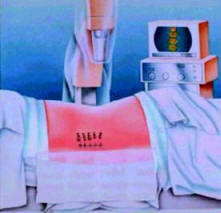
Figure-1 |
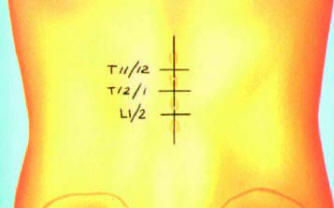
Figure-2 |
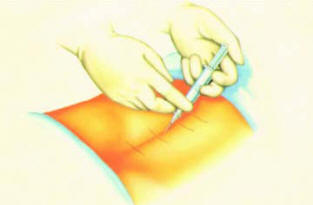
Figure-3 |
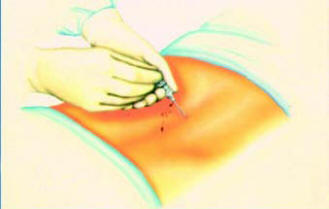
Figure-4 |

Figure-4 |
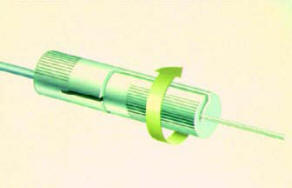
Figure-5 |

Figure-6 |
|
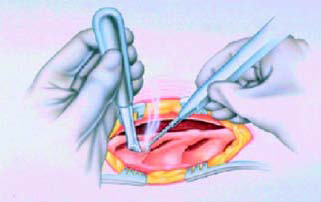
● Using a long needle to provide a marker at the midbody of T10
to provide a constant point of reference (Figure 2)
● Making a small puncture wound and inserting the Tuohy needle
under
fluoroscopic guidance (Figure 3)
● Introducing the lead and threading it to the target vertebra
(Figure-4)
● Attaching the screening cable to the proximal end of the lead
when the
lead is in place (Figure 4 and 5)
At this point the physician will
pass the end of the screening
cable out of the sterile field to
the nurse. With the screener
off, the nurse will connect the
cable to the screener
(temporary power source) and
intraoperative test stimulation
will begin (Figure 6).
 |
Surgical leads
Surgical lead placement is usually
performed under general anesthesia,
though sometimes under local anesthesia, within the sterile field. The
physician will begin the lead placement
procedure by:
● Identifying the vertebral body using
fluoroscopy and administering an
anesthetic along the incision site.
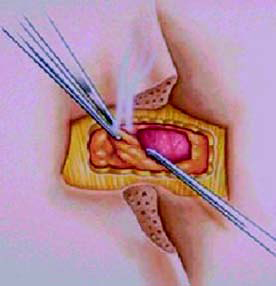
● Making the skin incision and then
using electrocautery to expose the
interlaminar space (Figure-7)
● Completing the laminectomy by
exposing the dura matter
(Figure-8)
● Introducing the lead blank and
then passing the lead through
the ligament window
(Figure-8 and 9)
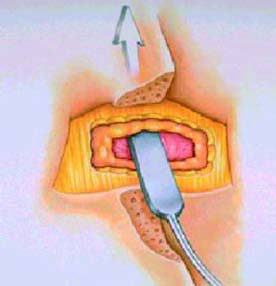
● Passing the lead cephalad (towards the head) until optimal
position
is achieved (Figure 10)
● At this point the physician will pass the end of the screening
cable out
of the sterile field to the nurse. With
the screener off, the nurse will connect
the cable to the screener (temporary
power source) and intraoperative test
stimulation will begin. There are two
different types of temporary screener,
one for the single lead and one for the
dual lead (Figure 11) |
 Intraoperative test stimulation
Intraoperative test stimulation
The purpose of intraoperative test stimulation is to create an
appropriate
field of paresthesia to cover the patient’s pain pattern. The
location and
strength of the paresthesia are optimized by manipulating:
● Lead position along the length of the
patient’s spinal cord
● Stimulation parameters (amplitude,
pulse width and rate)
● Lead electrode selection (i.e. changing
the location and number of anodes and cathodes on the lead).
 Screening test period
Screening test period
The basic screening protocol, which should be followed in every
diagnostic
test stimulation procedure, is carried out as follows:
● The screener is turned on and the
amplitude is set to 0 V, the pulse width to
210 msec and the rate to 40 Hz
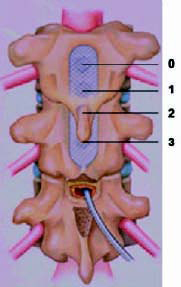
● To start, the centre bipole electrode
selection should always be used. Center
bipole consists of one positive electrode
and one negative electrode on each lead
(Figure 12)
● The amplitude is increased to the point
where the patient begins to feel a tingling
sensation
● The amplitude is increased until the
patient’s pain threshold (intensity of a noxious stimuli
necessary for a
person to perceive pain) is reached
● The amplitude is reduced to the point where the
patient feels comfortable, yet strong, paresthesia
● Microadjustments in lead position are made until the
optimal lead position is located (trolling)
● The pulse width is gradually adjusted until it covers
the breadth of the patient’s pain. Some patients with
back and leg pain may require a pulse width higher than
330 μs
● At the end of the screening period, all system
settings are carefully documented. Normally, amplitude
and pulse width are usually the only parameters adjusted
during intraoperative test stimulation. The rate should
remain at 40 Hz. |
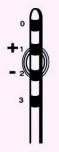
Figure-12: Center Bipole electrode selection. |
 Patient evaluation
Patient evaluation
Patient evaluation, which occurs between diagnostic test
stimulation procedure and complete system implant, may last from
a day to several weeks. During patient evaluation, patients
receive a screener with which to adjust (within a
clinician-defined range) the stimulation parameters established
during intraoperative test stimulation. Patients should fully
test the neurostimulation system under their normal, day-to-day
activities, such as walking, sitting and working. Patients are
encouraged to try different amplitudes to determine optimal
device settings. Some centers encourage patients to document the
setting after each use along with the duration of the
stimulation and time of day. The screening test period is also
an opportunity for the patient to develop an understanding of
the technology and realistic expectations of the therapy.
Patient evaluation allows the physician and the patient to:
● Evaluate the impact of stimulation on the patient’s pain and
daily life
● Optimize settings for the best pain relief
● Identify the patient’s energy requirements
● Determine whether the patient is comfortable both with the
operating system and with the feeling of paresthesia
If the patient does not respond positively to neurostimulation
during the screening test period, the lead is removed and the
patient is often referred for an intrathecal drug delivery
trial. However, if the patient experiences pain relief of at
least 50% during test stimulation, the physician and the patient
may agree to proceed with the implantation of a complete system.
 Complete system implant
Complete system implant
If the diagnostic test stimulation procedure was successful, a
complete
system can be implanted. Neurostimulator selection is based on
initial stimulation parameters, patient evaluation data and lead
requirements.
Complete system implant is usually scheduled two weeks after the
diagnostic test stimulation procedure to provide the patient
with ample
time to evaluate neurostimulation and to reduce the likelihood
of infection.
Possible implant sites include the upper abdominal wall, the
upper
buttocks, or a subclavicular (below the collar bone) pocket. The
choice of
implant site is determined by patient and physician preference.
The most
common site is the upper abdominal wall (Figure 13–16).
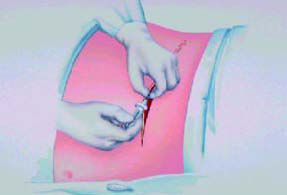
Figure-13 |
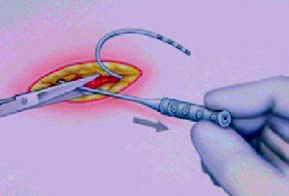
Figure-14 |
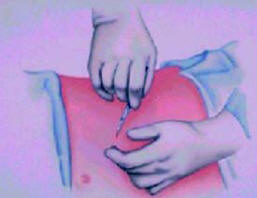
Figure-15 |

Figure-16 |
 Programming stimulation parameters
Programming stimulation parameters
Stimulation parameters are programmed according to patient
evaluation
data and the parameters determined during intraoperative test
stimulation.
There are two considerations with respect to device programming:
● Maximizing patient comfort
● Extending battery life (e.g. rates greater than 50 Hz
substantially shorten
battery life)
In order to extend battery life, some neurostimulators can be
programmed
to cycle. Cycling means that the device can be programmed to
turn on and
off for specified periods of time.
 Postoperative management
Postoperative management
Once the neurostimulation system is implanted, patients are
closely
monitored. Postoperative care involves:
● Management of complications
● Patient education
● Care of the operative site
 Management of complications
Management of complications
Potential postoperative complications include:
● Surgical complications
● System complications
 Surgical complications
Surgical complications
Patients who have poor nutritional status, are small in build
and/or thin, or
who have generally poor health are at greater risk for
post-surgical
infections. Potential surgical complications include:
● Infection
● Hematoma
● Epidural hemorrhage
● CSF leakage
● Pain and discomfort
● Seroma at the neurostimulator or receiver site
 System complications
System complications
Complications with a neurostimulator system may occur and
include:
● Battery failure and/or battery
leakage
● Lead and/or neurostimulator erosion or migration
● Connection problem
● Wire breakage
● Short circuit
In addition, the patient may experience:
● Loss of pain relief
● Loss of stimulation
● Undesirable change in stimulation described by some patients
as
uncomfortable ‘jolting’ or ‘shocking’
● Radicular chest wall stimulation
● Allergic or immune system response to the implanted materials
 During the postoperative period, patients are advised to limit
their activities
to reduce the risk of lead movement and subsequent loss of
stimulation
effectiveness. Postimplant instructions are listed in the table
below.
During the postoperative period, patients are advised to limit
their activities
to reduce the risk of lead movement and subsequent loss of
stimulation
effectiveness. Postimplant instructions are listed in the table
below.
|
Table 2: Postimplant instructions for SCS patients |
|
To help reduce lead movement and subsequent loss of stimulation,
patients must
follow the activity precautions listed below. |
| Immediately after surgery |
● Patients should stay in bed for 10
to 12 hours or
according to their physicians instructions. Bed
rest reduces the risk of lead movement
● Patients should raise the head of their bed by
around 20 degrees for the first postoperative
night to support their spine |
| The day after surgery |
● Patients should walk for brief periods
to build up
their physical strength, keeping their back as
straight as possible to prevent lead movement |
| For 6 to 8 weeks after
surgery |
PATIENTS SHOULD NOT:
● Put their arms over their head
● Bend, twist, stretch or lift more than 2.5kg
● Sleep on their stomach
● Climb too many stairs
● Sit too long in a chair
● Drive for several weeks to reduce the risk of
abrupt movements or shifts in position which will
increase the risk of lead movement
● Operate motor vehicles, power tolls or equipment
while the stimulator is on.
PATIENTS SHOULD:
● Sleep with a firm mattress
● Resume showering and bathing
● Follow their physician’s recommendations
regarding sexual activity
● Obtain approval from their physician before
having their spine manipulated by a chiropractor
or other physician
● Move the upper part of their trunk as one i.e. the
head should not be turned without also turning
the hips
● Build up their physical strength by walking for
brief periods of time each day or engaging in a
physical therapy program according to their
physicians instructions |
As well as the above postimplant
instructions, there are also many ongoing
instructions and precautions that all patients should follow.
These differ
depending on whether the patient has received an IPG system or
an RF
system. Special instructions and precautions for both systems
are shown in
tables 3 and 4.
|
Table 3: Special instructions and precautions for patients with
a battery (IPG) system |
| For patients with control magnets: |
| ● The control magnet should be kept away from the implanted
battery when it is not
being used to turn the stimulator on or off. This is because the
magnet may turn
the neurostimulator on or off. |
| For patients with patient programmers: |
● Patients should exercise caution when increasing amplitude to
avoid unpleasant
stimulation e.g. ‘shocking’ or ‘jolting’. Patients should always
start at the lowest
amplitude and then slowly increase
● The programmer and its accessories should be cleaned with a
damp sponge or
cloth moistened with water, mild detergent or alcohol. Patients
should not allow
excessive moisture into the programmer
● If patients use the optional antenna with their programmer,
they should follow the
skin care instructions outlined for the RF-transmitter antenna |
| To avoid damaging the programmer patients should not: |
● Immerse the programmer into any liquid
● Clean the programmer with aggressive cleaning agents such as
paint thinners,
turpentine or nail polish remover |
|
Table 4: Special instructions and precautions for patients with
an RF system |
| Care of the skin around the RF transmitter antenna: |
● To reduce the risk of skin irritation in the area of the
antenna, patients should keep
the skin in that area clean, dry and free of pressure
● Patients should inspect the skin area daily where they place
their antenna to ensure
that it is healthy. If there is any swelling or redness in the
area, patients should
contact their physician before using the antenna again
● Each day patients should clean the skin over the receiver with
an antibacterial soap
and change the antenna attachment discs. |
| Care of the RF transmitter and antenna: |
● Each week patients should clean the outer surface of their
transmitter using a
cloth dampened, but not saturated, with mild soap and water.
Never dip the
transmitter in cleaning solution
● Every day, patients should wash their antenna with mild soap
and water. Before
cleaning, the antenna should be disconnected from the
transmitter. The antenna
should be rinsed thoroughly to eliminate any soap. Patients must
be careful to
avoid getting the metal connector end of the cord wet. The
antenna should be
dried with a clean towel immediately after washing. When not in
use the antenna
should be stored in a plastic bag to protect it from dust
● Patients must replace the battery when the BAT indicator
appears on the display,
if they suspect weak batteries, or if they do not get adequate
pain relief from the
highest amplitude setting. To prevent corroding the electronic
components,
patients must not leave depleted batteries in the transmitter
● The transmitter should be stored at room temperature. Extremes
of hot and cold
or direct sunlight should be avoided. If the transmitter is
stored for more than a
month, the 9 V battery should be removed |
|














 What’s Up
What’s Up