|
 INTRODUCTION
INTRODUCTION
The full potential of intraoperative neurophysiology is
realized during the performance of so-called functional
neurosurgical procedures. During these interventions
therapeutic lesions or stimulating electrodes are
stereotactically placed within deep brain structures to
treat movement disorders such as Parkinson’s disease (PD),
essential tremor (ET), dystonia, affective disorders, and
chronic neuropathic pain.
The deep location of these structures precludes direct
surgical approaches. Instead, surgeons rely on a combination
of image-guided stereotactic techniques and intraoperative
neurophysiology to place the therapeutic lesions or
stimulating electrodes with acceptable accuracy and safety.
Unlike tumors, which are relatively large and easily
identified on CT or MRI, functional neurosurgical targets
typically are small and poorly visualized with current
imaging modalities. Moreover, because these are physiologic
as much as anatomic targets, image-based targeting may
incompletely identify the desired location. Consequently,
intraoperative recording and stimulation techniques have
been developed to aid target localization. These techniques
complement anatomical targeting by providing real-time
electrophysiological data concerning probe position and the
surgical target. The surgeon and physiologist use these data
to “fine-tune” their anatomic targeting before completing
the therapeutic intervention. Thus employed, intraoperative
neurophysiology does not simply monitor surgical activity;
it guides it.
 Surgical History of Movement Disorders
Surgical History of Movement Disorders
Sir Victor Horsely is reported to have performed the first
neurosurgical procedure for a movement disorder when, in the
late 1800s, he resected part of the precentral gyrus in a
patient with athetoid movements. The surgery halted the
abnormal movements but caused dyspraxia and paralysis of the
limb.
The first successful basal ganglia surgery is credited to
Meyers, who reported improvement in a patient with
postencephalitic parkinsonism in 1939. Prior to this
landmark report, surgery within the basal ganglia was
avoided because it was believed that human consciousness
resided in these structures. Despite the high mortality
rates (10–12%) that plagued these “open” procedures (i.e.,
via craniotomy), Meyers demonstrated the potential benefits
of basal ganglia surgery and opened the door for the
application of less invasive stereotactic approaches to
these deep brain structures. He also provided the first
accounts of human basal ganglia physiology, describing the
frequency, phase, and amplitude of neuronal signals from the
striatum, pallidum, corpus callosum, internal capsule,
subcallosal bundle, and dorsal thalamus in patients with and
without movement disorders. Meyers quickly realized the
potential value of the accumulated data, which he ultimately
employed to help localize specific deep brain structures
during movement disorder surgery.
Robert Clarke designed the first stereotactic frame in 1908.
His frame employed skull landmarks to target deep brain
structures in small animals, a technique that could not be
translated to clinical use because of the more varied and
complex shape of the human skull and brain. Consequently, it
was not until 1947, after the introduction of
ventriculography, that Spiegel and Wycis performed the first
human stereotactic surgeries, for psychiatric illness and
Huntington’s chorea. In following years a number of human
stereotactic atlases were published, and standard meridia
(e.g., the intercommissural line) from which stereotactic
coordinates could be determined were established.
Effective targets for stereotactically guided neuroablation
were discovered empirically. For example, Cooper stumbled
upon the beneficial effects of globus pallidus lesioning by
accidentally ligating the anterior choroidal artery of a PD
patient while performing a pedunculotomy. He later adopted
stereotactic approaches to pallidal lesioning, reporting
favorable results and reduced surgical mortality rates (∼3%)
as compared to open procedures. Laitinen described how
Leksell further improved the results of pallidotomy by
placing the lesion more posteriorly and ventrally within the
internal
segment of the globus pallidus (GPi), that portion of the
nucleus that we now know is responsible for sensorimotor
processing. In 1963, Spiegel et al. described campotomy, in
which the fibers of the pallidofugal, rubrothalamic,
corticofugal, and hypothalamofugal pathways are interrupted
within the H fields of Forel. They reported promising
results in 25 patients with tremor and 25 with rigidity. In
the end, however, thalamotomy emerged as the most commonly
performed movement disorder procedure in the pre-levodopa
era because of the consistent tremor control it provided.
Though most surgery for PD ceased after the introduction of
levodopa in 1967, small numbers of thalamotomies were
performed for medically refractory tremor during the next 25
years, until the reintroduction of Leksell’s pallidotomy by
Laitinen et al. in 1992.
 Neurophysiology and Movement Disorder Surgery
Neurophysiology and Movement Disorder Surgery
Most early electrophysiologic studies of the human thalamus
and basal ganglia were performed with macroelectrode
techniques that yielded relatively crude, EEG-like
responses. Electrodes and recording techniques were refined
over subsequent decades, culminating in the development of
single-cell microelectrode recording. Of note is the work of
Albe-Fessard, who refined microelectrode techniques for
experimental purposes and paved the way for their
intraoperative use. It was her belief that micro-electrode
recording (MER) would “provide a powerful tool in improving
stereotactic localization and that it would furthermore
reduce the risk due to anatomical variability”. In recent
years, Madame Albe-Fessard’s vision has been realized as MER
has gained in popularity and ready-to-use recording systems
have become commercially available.
The history of electrical brain stimulation begins with
Fritsch and Hitzig, who in 1870 elicited limb movement in
dogs by stimulating the frontal cortex, and then defined the
limits of the motor area electrophysiologically.
Intraoperative cortical stimulation studies by Penfield and
colleagues from the late 1920s through the late 1940s
contributed seminal information concerning the somatotopic
organization of the cerebral cortex by defining the motor
and sensory “homunculi.” In 1950, Spiegel et al. described
the use of stimulation during surgery at the H fields of
Forel to both “test the position of the electrode and to
avoid proximity to the corticospinal pathways ventrally, the
sensory thalamic-relay nuclei dorsally, and the third
nucleus posteriorly”.
Other neurophysiological techniques, such as impedance
monitoring and evoked potential recordings also have been
employed as localization tools; however, these techniques
serve predominantly as adjuncts to recording and
stimulation.
Perhaps the most significant advance in functional
neurosurgery in the last decade has been the introduction of
chronic electrical stimulation (termed “deep brain
stimulation” or DBS) as a therapeutic alternative to
neuroablation.
Deep brain stimulation provides three potential advantages
when compared to neuroablation:
1. DBS is reversible. If stimulation induces an unwanted
side-effect, one simply turns the stimulator off or adjusts
parameters. Thus the risk of permanent adverse neurological
events is reduced.
2. Stimulation parameters may be customized to each patient,
potentially enhancing therapeutic efficacy.
3. Access to the surgical target is maintained via the
implanted electrode and programmable pulse generator.
Therefore, therapy may be modified over time through simple
stimulation adjustments, potentially increasing the
longevity of response.
| |
 |
|
| |
FIGURE.1 A three-dimensional artist’s rendition of the
structures involved in surgery for
movement disorders. The light greenish blue structure on the
left is the globus pallidus (GPi and
GPe). The large grey structure on the right is the thalamus,
and the small dark green structure is
the subthalamic nuclei (STN). The medial edge of the STN is
only 6.0 mm from the midline of the brain and around
10.0 mm for GPi, 11.0 mm and for VIM. |
|
Thus far, two studies that compared thalamic DBS to
thalamotomy for the
treatment of tremor have been published. Both studies found
DBS to be the
superior treatment modality in large part because of the
ability to adjust stimulation
parameters in the event of symptom recurrence.
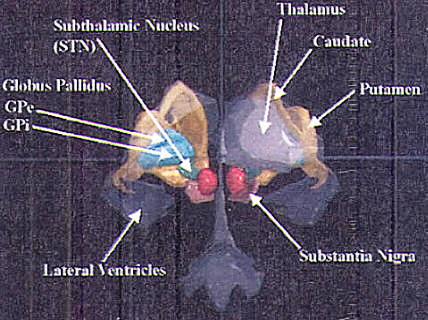
Presently, movement disorder surgery is focused on three
structures: the
ventrolateral (VL) nucleus of the thalamus, the globus
pallidus pars internus
(GPi), and the subthalamic nucleus (STN) (Fig.1).
Each of these structures can be targeted for ablation in
procedures that are,
respectively, termed thalamotomy, pallidotomy, and subthalamotomy. Alternatively, each can
be targeted for
chronic electrical stimulation. The
choice of target is based largely on clinical diagnosis and
the symptoms to be treated.
 THEORETICAL BASIS FOR SURGERY
IN THE BASAL GANGLIA
THEORETICAL BASIS FOR SURGERY
IN THE BASAL GANGLIA
Our current understanding of the functional organization of
the basal ganglia
and PD pathophysiology is based predominantly on data
derived from the study
of primates with
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced
Parkinsonism. Microelectrode techniques also have
contributed
greatly to this body of knowledge. Though incomplete, the
current model of
basal ganglia function is partly responsible for the rebirth
of movement disorder
surgery, providing a scientific basis for selecting those
deep brain structures
that are currently targeted for therapeutic interventions.
The model is depicted in Fig.2. The basal ganglia are
composed of two principal
input structures (the corpus striatum and the STN), two
output structures
(GPi and substantia nigra pars reticulata [SNr]), and two
intrinsic nuclei (external
segment of the globus pallidus [GPe] and substantia nigra
pars compacta [SNc]). Five parallel basal ganglia-thalamo-cortical circuits
(motor, oculomotor,
two prefrontal, and limbic) have been described. While
surgical interventions
target the motor circuit, it is likely that lesioning and
stimulation also impact other
circuits as well.
| |
 |
|
| |
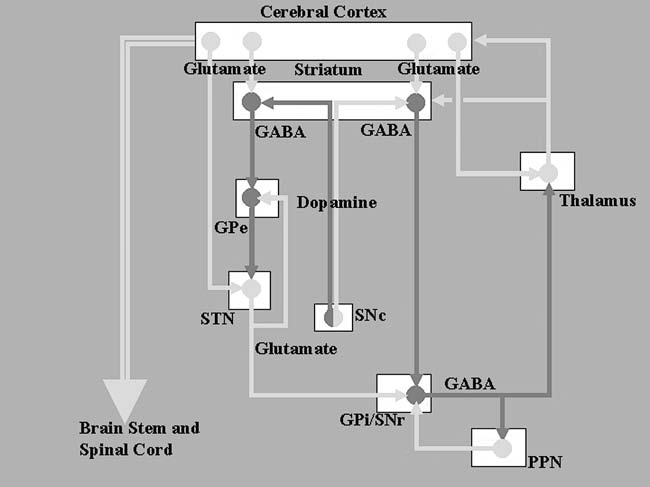
FIGURE.2 Diagrammatic representation of the basal ganglia
circuit, showing the direct and indirect pathways. The light grey lines represent
excitatory pathways, and the darker lines show inhibitory
pathways. |
|
 The corpus striatum, which is composed of the caudate and
putamen, is the
largest nuclear complex of the basal ganglia. The striatum
receives excitatory
(glutamatergic) input from several areas of the cerebral
cortex as well as
inhibitory input from the dopaminergic cells of the SNc.
Cortical and nigral
inputs are received via the “spiny” neurons. One subset of
these cells projects
directly to the GPi, forming the “direct pathway,” while
another subset projects
to the GPe, the first relay station of a complementary
“indirect pathway,”
that passes through the STN before terminating at GPi. The
antagonistic
actions of the direct and indirect pathways regulate the
neuronal activity of
GPi, which, in turn, provides inhibitory input to the
pedunculopontine
nucleus (PPN) and the VL nucleus of the thalamus. The VL
nucleus projects
back to the primary and supplementary motor areas, completing
the cortico-ganglio-thalamo-cortical loop. The direct
pathway inhibits GPi,
resulting in a net disinhibition of the motor thalamus and
facilitation of the
thalamo-cortical projections. The indirect pathway, via its
serial connections,
provides excitatory input to the GPi, inhibiting the thalamo-cortical
motor
pathway.
The corpus striatum, which is composed of the caudate and
putamen, is the
largest nuclear complex of the basal ganglia. The striatum
receives excitatory
(glutamatergic) input from several areas of the cerebral
cortex as well as
inhibitory input from the dopaminergic cells of the SNc.
Cortical and nigral
inputs are received via the “spiny” neurons. One subset of
these cells projects
directly to the GPi, forming the “direct pathway,” while
another subset projects
to the GPe, the first relay station of a complementary
“indirect pathway,”
that passes through the STN before terminating at GPi. The
antagonistic
actions of the direct and indirect pathways regulate the
neuronal activity of
GPi, which, in turn, provides inhibitory input to the
pedunculopontine
nucleus (PPN) and the VL nucleus of the thalamus. The VL
nucleus projects
back to the primary and supplementary motor areas, completing
the cortico-ganglio-thalamo-cortical loop. The direct
pathway inhibits GPi,
resulting in a net disinhibition of the motor thalamus and
facilitation of the
thalamo-cortical projections. The indirect pathway, via its
serial connections,
provides excitatory input to the GPi, inhibiting the thalamo-cortical
motor
pathway.
 In PD, loss of dopaminergic input to the striatum leads to a
functional reduction
of direct pathway activity and a facilitation of the
indirect pathway. These
changes result in a net increase in GPi excitation and a
concomitant hyperinhibition
of the motor thalamus. The excessive inhibitory outflow from
GPi
reduces the thalamic output to supplementary motor areas
that are critical to
the normal execution of movement.
In PD, loss of dopaminergic input to the striatum leads to a
functional reduction
of direct pathway activity and a facilitation of the
indirect pathway. These
changes result in a net increase in GPi excitation and a
concomitant hyperinhibition
of the motor thalamus. The excessive inhibitory outflow from
GPi
reduces the thalamic output to supplementary motor areas
that are critical to
the normal execution of movement.
This model accounts well for the negative symptoms of PD
(i.e., rigidity and
bradykinesia) and supports both GPi and STN as rational
targets for surgically
treating PD. The model is incomplete, however, because it
does not fully
account for hyperkinetic features of PD such as tremor and
levodopa-induced
dyskinesias, physiological phenomena that are poorly
understood.
Tremor activity is consistently detected in the VL nucleus
of patients with
PD or ET, and the VL nucleus continues to be the primary
surgical target for
treating medically refractory tremor. However, it is unclear
if the motor thalamus
is the primary generator of tremor activity or merely
participates in the
transmission of tremor-generating signals. Moreover, the
evidence that both
pallidotomy and STN DBS also control parkinsonian tremor suggests
that intervention at many points within the
tremor-generating loop may
suppress this symptom.
Levodopa-induced dyskinesias (LIDs) are involuntary
movements of the limbs
or trunk that are temporally associated with levodopa
administration.
These movements are typically choreiform or dystonic in
nature and are easily
distinguished from the tremor of PD. Pharmacodynamic factors
related to
chronic exogenous dopaminergic stimulation probably play a
fundamental role
in levodopa-induced dyskinesia. According to the model,
pallidotomy should
worsen LID by reducing pallidal inhibition of the VL
nucleus, a hypothesis that
is supported by the experimental observation that STN
lesions, which reduce
the excitatory output from STN to GPi, cause dyskinesias in
primates that are
indistinguishable from LID. On the contrary, LID is
the most responsive
symptom to pallidotomy, a consistently observed phenomenon. It
has been hypothesized that sensitization of dopamine
receptors by exogenously
administered levodopa may cause aberrant neuronal firing
patterns with consequent
disruption of the normal flow of information to the thalamus
and the
cortical motor areas. It follows that pallidotomy may
improve LID by disrupting
this aberrant flow.
 MOVEMENT DISORDER SURGERY:
MOVEMENT DISORDER SURGERY:
 GENERAL OVERVIEW
GENERAL OVERVIEW
There is no one best method for performing movement disorder
surgery.
Rather, stereotactic surgeons modify general approaches to
target localization
to suit their personal preferences and to take advantage of
their institution’s
strengths. Currently accepted technique involves frame-based
anatomical localization
supported by intraoperative physiological confirmation of
proper targeting.
 Anatomical Targeting Techniques
Anatomical Targeting Techniques
In the pre-levodopa era, positive contrast and air
ventriculography were
employed to localize the foramen of Monro and the anterior
and posterior
commissures. The stereotactic coordinates of therapeutic
targets were then
determined based on their relationship to these
structures as described
in various stereotactic atlases. Targeting accuracy was
therefore limited by the
inaccuracies of these atlases, which were typically
generated from just one or
a few specimens whose true dimensions were distorted by
formalin fixation
and by anatomical distortions created by the
intraventricular injection of air
or contrast. Today, CT- and MRI-based techniques, which
demonstrate the
brain parenchyma noninvasively, have supplanted
ventriculography as the
primary means of anatomically localizing stereotactic
targets. Nevertheless,
ventriculography is still employed by many stereotactic
surgeons and therefore
remains an important technique.
The introduction of CT revolutionized the diagnosis
and treatment of
neurologic diseases and encouraged changes in stereotactic
frame design,
expanding the uses of frame-based stereotaxis to include
tumor biopsy and
resection. Soon after the introduction of MRI, Leksell et
al.
demonstrated its applicability to stereotactic systems. MRI
provides superior
resolution as compared to CT, as well as multiplanar images
with
minimal frame-related artifact. Nonreformatted MRI
beautifully demonstrates
the commissures, the thalamus, and most basal ganglia
structures.
These features permit direct stereotactic localization of
the surgical target in
some instances; however, indirect targeting,
based on accurate
localization of the commissures, may still yield the most
reliable target coordinates.
The most significant drawback to targeting with MRI is the
potential for
image distortion introduced by nonlinearities within the
magnetic field.
Distortions can be generated by a number of factors,
including the presence of
ferromagnetic objects within the field, imperfections in the
scanner’s magnets,
and, most commonly, patient movement. Walton
et al. demonstrated
that targeting errors are greater in the periphery than in
the center of the
magnetic field and stereotactic space. MRI
distortion may also be
related to the pulse sequence(s) employed. For example, it
has been suggested
that fast spin-echo inversion recovery sequences resist
imaging distortions secondary
to magnetic susceptibility better than other image
acquisition methods.
In contrast to MRI, CT maintains linear accuracy, thereby
reducing image-induced
targeting errors. However, metallic artifact can
impede visualization
of the commissures, CT tissue resolution is inferior to MRI,
and axial
images alone are provided. Commercially available targeting
software packages
can fuse CT and MRI images, but to our knowledge there are
no studies to suggest
that such image fusion techniques improve targeting
accuracy.
 Physiological Targeting: Recording Techniques
Physiological Targeting: Recording Techniques
The four most commonly employed techniques for physiologic
localization
during movement disorder surgery are: (1) impedance
measurements; (2)
macroelectrode recordings and stimulation; (3)
semimicroelectrode recording
(and/or stimulation); and (4) microelectrode recording (with
or without stimulation).
Evoked potentials have also been employed at times,
but at
present these are primarily used as an adjunct to
stimulation during thalamic
interventions.
 Impedance Techniques
Impedance Techniques
Changes in electrical impedance can accurately demarcate the
boundaries of
neural structures and may therefore be used to define the
borders of a surgical
target. Impedance measurements can be performed
with monopolar
electrodes that are referenced to the scalp or with
concentric bipolar
electrodes employing the outer ring as the reference. Employing a
test frequency of 1 KHz, impedances of 400 Ω or greater are
recorded in the deep
grey matter while white matter can be greater or less
depending upon orientation.
The major advantages of this technique are the ease with
which it is performed
and the fact that the same electrode can be used during both
the localization
and the lesioning phases of an ablative procedure. The major
disadvantage
is the relative crudeness of the physiological information
provided. Moreover,
impedance measures may not adequately distinguish borders
between adjacent
nuclei and work best when there are clear grey matter–white
matter boundaries
to be defined. Therefore, impedance recordings primarily are
used for the localization
of large white matter bundles and nuclear groups.
We perform
impedance measurements during ablative procedures only after
the final target
is selected via microelectrode recording, and simply to
ensure that the lesioning electrode has not strayed from its
desired trajectory
and is located within grey matter.
 Macroelectrode Recording
Macroelectrode Recording
Macroelectrode (ME) recording, defined as any low-impedance
(1–100 kΩ)
recording that generates either multiunit potentials or
neural background noise,
provides somewhat more detailed physiologic information as
compared to impedance
measurements. The electrode tip may be as
small as 50 μm
and may be configured in a bipolar concentric fashion with
an intertip distance
of 200–300 μm, or as a single active tip referenced to the
cortical surface via the
insertion cannula or to the scalp via a surface electrode.
The main advantage of ME recording is the ease and speed
with which data
are collected as compared to microelectrode techniques. The
obvious disadvantage
is that EEG-like field potentials lack the discrimination
necessary to
characterize single-unit firing features within the surgical
target (Fig.3).
Consequently, physiologic detail regarding the surgical
target is lacking.
| |
 |
|
| |
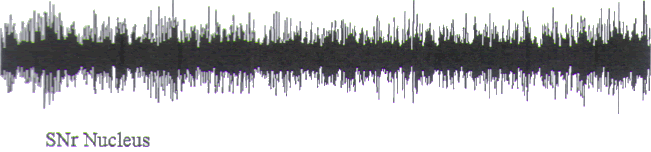
FIGURE.3 This is a poor semi-microelectrode recording
from a substantia nigra pars reticulata
cell. Note the multiple amplitude activity and the depth of
EEG quality. This cell was recorded from
an electrode that had an impedance of around 50 kΩ. The
diameter of the electrode was around
50 μm (5 s epoch). |
|
 Semi-Microelectrode Technique
Semi-Microelectrode Technique
Electrodes that have small tip diameters (<50 μm) and
impedances of
100–500 kΩ are referred to as semi-microelectrodes. These
electrodes provide
more detailed information than do macroelectrodes, but they
still do not yield
single-unit recordings. Semi-microelectrodes detect the
responses of a few cells
(∼10−100) (Fig.4) localized to a small area around the
recording tip (∼10−
100 μm). These so-called field potentials are more refined
than the EEG-like
recordings provided by macroelectrodes but lack the detail
provided by microelectrode
techniques.
| |
 |
|
| |
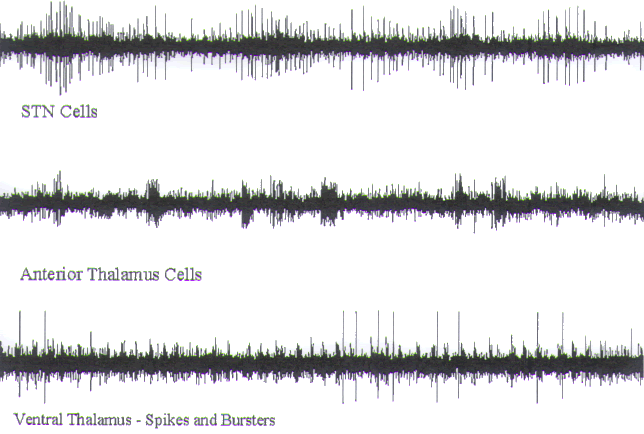
FIGURE.4 Three semi-microelectrode recordings in which
single units can be distinguished.
What differentiates these from pure microelectrode
recordings is the fact that they contain more
than one clearly distinguishable unit (5 s epoch). |
|
 Microelectrode Techniques
Microelectrode Techniques
Microelectrodes provide the most detailed picture of the
neural elements encountered during movement disorder surgery. Microelectrode tips have diameters of 1–40 μm
and impedances
of ∼1 MΩ. By recording individual neuronal activity (Fig.5), microelectrodes
provide real-time information concerning the physiological
characteristics of the
recorded neuron and thereby the nucleus within which the
cell is located.
The major drawback to microelectrode recordings is the time
and expertise
required to perform the technique well. The sophisticated
electronics equipment
is expensive and must be maintained expertly. Thus the
investment in
machinery and personnel can be prohibitive to some centers.
It is sometimes
difficult to acquire a useful signal because of electrical
noise in the operating
room, and even in the best circumstances, recording tracts
may take 20–40 min
to complete. Finally, interpreting single-cell recordings is
a skill that is mastered
only with experience and patience. Once
mastered, microelectrode recording can be performed
efficiently and yields
invaluable data concerning electrode position. For example,
Alterman et al.
demonstrated that in 12% of 132 consecutive pallidotomies,
final lesion placement,
as guided by microelectrode recording, was more than 4 mm
removed
from the site that was originally selected by the surgeon
based on stereotactic
MRI. This distance is considered significant, since it
is equivalent to the
diameter of the typical pallidotomy lesion.
| |
 |
|
| |
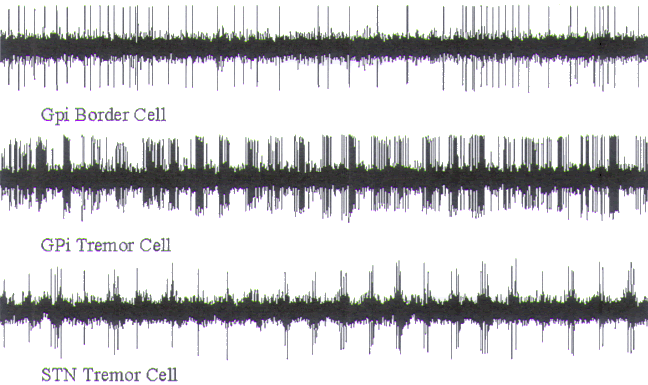
FIGURE.5 A set of microelectrode recordings. Note that
only a single unit is being recorded.
Each spike has relatively the same amplitude and shape (5 s
epoch). |
|
 OPERATING ROOM ENVIRONMENT
AND BASIC EQUIPMENT
OPERATING ROOM ENVIRONMENT
AND BASIC EQUIPMENT
OPERATING ROOM
 Electrical Noise (recording only)
Electrical Noise (recording only)
It is difficult to record low-amplitude neural signals
reliably in the electrically
harsh operating room environment, which can affect even more
robust, easily
recorded signals, such as the EKG. Anesthesia equipment,
electric cautery,
lighting, radios, telemetry equipment, and countless other
electronic devices
can all negatively impact recording quality. While the
surgical team can control
the use of these devices within their own operating room,
external electrical
influences, such as ongoing construction, poor wiring, and
the use of large
pieces of equipment in adjacent operating rooms, may also
erode recordings. In
order to control for these external influences fully,
movement disorder surgery
procedures ideally should be performed in an electrically
shielded operating
room. Of course, few facilities possess such an expensive
facility, so we make
the following recommendations:
1. Minimize any stray electrical switching noises.
Typically, this type of noise
derives from two sources: lighting fixtures that are
equipped with dimmers
and poorly shielded computer equipment. A
properly
grounded recording head stage can be operated with minimal
switching
interference when the dimmers are set either all the way on
or all the way off.
Fluorescent lighting may also interfere with the recording
equipment, but such
60-Hz signals are attenuated easily with a notch filter.
Computer monitors
should be fitted with static screen covers that can be
grounded. If the monitor
is part of the recording system, it can be grounded to the
common system
ground. Otherwise, it should be grounded to the operating
room grounding
system.
2. Employ battery-powered anesthesia and monitoring
equipment. Alternatively,
position anesthesia equipment in such a way as to reduce
electrical interference.
Turn down audible indicators. One can reduce cross-talk by
keeping monitoring
and neural recording cables on opposite sides of the
patient. Newer anesthesia
systems are equipped with cathode ray tube (CRT), liquid
crystal display (LCD),
and/or plasma displays, the electromagnetic (EM) leakage
from which can be
bothersome. If the interference from such monitors becomes
overpowering, a
simple aluminum foil shield can be placed between the
monitor and the recording
stage and connected to the system ground.
3. Turn off and unplug all electrical equipment that is not
in use during
recording. Electric cautery, electrically controlled
operating tables, and patient
warmers generate very powerful electromagnetic radiation.
Fortunately, these
devices are not necessary during recording and can be
unplugged.
4. Employ isolated power supplies for the recording
equipment. Electrical equipment
used in adjacent operating rooms may interfere with
recording due to
poor operating room wiring schemes. Employing isolated power
supplies and
grounded EM shields can minimize this interference.
Proper planning will help minimize most sources of noise,
but noise will
occur despite the most prudent planning. It is important
that both the surgeon
and the neurophysiologist are prepared for these occasional
frustrations. The
patient should also be informed of the possibility of delays
during the surgery
should electrical noise be encountered. Taking the
aforementioned preventive
steps minimizes the risk of encountering noise and provides
a framework from
which one can troubleshoot noise problems when they occur.
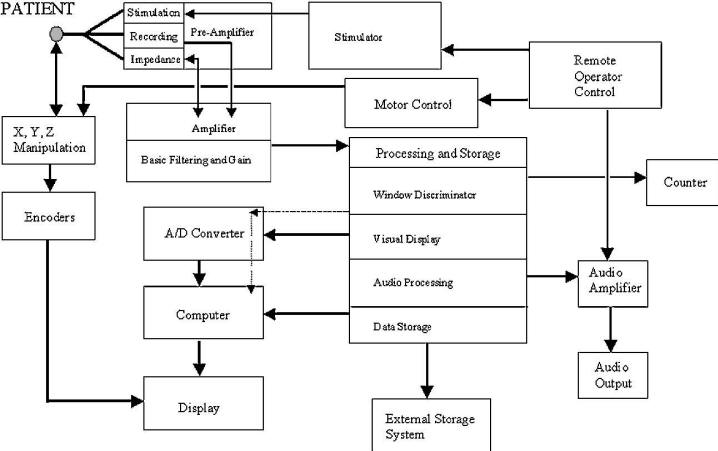
 Electrical Noise (internal system influences)
Electrical Noise (internal system influences)
Sources of electrical noise from within the recording system
include: (1) the
microelectrode transducer, which detects the neural
activity; (2) the preamplifier,
which is located close to the recording structure; (3) the
amplifier; (4) signal
conditioners; (5) the visual display; and (6) auditory
processors (Fig.6).
| |
 |
|
| |
FIGURE.6 A representation of the signal flow through the
intraoperative recording system.
The microelectrode (or transducer) converts the cellular
chemical potentials to a pure electrical
signal that is then passed though the amplification system.
From there the data pass through a digitizer
or audio processing system. The data are then displayed on a
computer, amplified and played
through audio speakers, and stored for off-line analysis. |
|
However, electrical noise primarily enters the system
proximal to the first stage
of the preamplifier.
The amplitude of the recorded signals is small (range: 100
μV to 100 mV) so
that failure of any real-time component can severely
compromise the integrity
of the signal and, in turn, the accuracy of the mapping.
Poorly designed equipment
is the most common cause of intrasystem noise; poor system
maintenance
is second. Connectors must be cleaned or replaced regularly
to combat oxidation,
particularly in high-humidity environments. Cables must also
be inspected
regularly and replaced when worn.
 RECORDING ELECTRODES
RECORDING ELECTRODES
Lenz has previously described the construction of
recording microelectrodes,
and Geddes provides a useful description of electrode
properties.
Microelectrode tips may be composed of a number of
materials, including
stainless steel and tungsten, but the authors prefer the
platinum-iridium
etched tip, which is glass coated. The tip diameter ranges
from 1 to 40 μm and
is beveled to a maximum diameter of 350–400 μm. The tip is
coated with a thin
layer of glass to make the maximum diameter between 400 and
450 μm. The
electrode tip is connected to a stainless steel wire
(diameter: 500 μm) and a
glass soldered bead. Alternatively, Epoxylite is used to seal the junction. An outer insulating
sheath is placed
over the stainless steel wire, making the total shaft
diameter 600–700 μm. An
electrode (including the tip) is typically around 300 mm in
length. The last
15–20 mm of insulation is removed in order to connect the
electrode to the
amplifier.
The electrodes exhibit a low-frequency roll-off below 1000
Hz (Fig.7).
| |
 |
|
| |
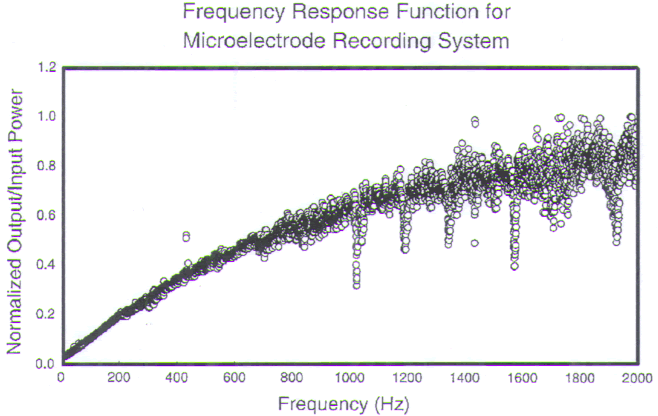
FIGURE.7 The gain versus frequency of the recording
system. The recording system acts as a
high-pass filter. Below 1000 Hz there is a reduction in the
system’s gain. This reduction is acceptable because most of
the spike energy is contained in the higher
frequencies of the spike. |
|
The resulting reduction in transmitted power (frequency
range: 100–2000 Hz)
can be as much as 17.9 dB [161]. Even though cellular firing
rates range from
5 to 500 Hz, it is the high-frequency components that are
most important for
auditory discrimination. The microelectrodes exhibit
adequate response characteristics
in these higher frequencies.
Semi-microelectrodes are usually made of either stainless
steel or tungsten
with tip diameters of less than 50 μm; however, tip
impedance and geometry
impact recording discrimination (i.e., field potentials vs.
single unit recordings)
more than tip diameter. Semi-microelectrodes are
technically easier to
produce than microelectrodes because they can be made from
existing fine wire,
while microelectrode tips must be electrolytically etched.
 AMPLIFICATION
AMPLIFICATION
The preamplifier is the first active component of the
recording system. Either
referential or differential amplification techniques are
employed to measure
voltage variations at both the active and referential
inputs. Referential amplifiers
reference the active input to a second input that is either
located far from
the active input and/or has a larger surface area than the
active input. The variations
measured by the active input are independent of the
relatively inactive
reference input, permitting discrimination of the true
signal. In reality, large
amplitude signals in the reference electrode may conceal
smaller voltage variations
at the active electrode, masking signal. This possibility
should be kept
in mind if extensive noise is observed on the recording
display. Another disadvantage
of referential recording is the possibility of amplifying
noise that is
mistakenly interpreted as signal. Differential amplification
is superior in this
regard.
Differential amplifiers use two active inputs and
electrically subtract signals
that are common to both. The transmission cables of both
inputs run to the
amplifier side by side. The amplifier receives two of the
same input signal, but
one is 180° out of phase from the other (i.e., one is
positive while the other is
negative). The two active signals are then subtracted. Noise
that is externally
induced on the transmission cable is subtracted out since
the same noise is theoretically
induced in both the transmission and reference cables.
Differences in
the signals are also accentuated (SA1 + N − (−SA1 + N) = 2
SA1). The commonmode-
rejection-ratio (CMRR) defines the ability of a differential
amplifier to
exclude common input noise. The larger this number, the
greater is the reduction
of the induced cable noise.
Shils J.L. et al. employ a differential amplifier for intraoperative single-unit recording.
The ground
and the active input are interconnected on a large ground
plane to minimize
voltage variations, which are typically close to zero. The
ground plane includes
the head stage, the cerebral cortex, and the base of the
isolated amplifier. Isolation
is important for safety. These amplifiers must have a very
high input
impedance (∼200 MΩ) to enhance signal transfer from the
high-impedance
electrode (∼1 MΩ). Considering the electrode and amplifier
as a voltage
divider, the voltage at the amplifier is
determined by the equation:
As the amplifier input impedance approaches the electrode
impedance, signal
transfer decreases. The amplifier output impedance is 10 Ω.
The amplifier consists
of two sections: the preamplifier and a built-in impedance
test circuit.
The preamplifier attaches to the head stage and serves not
only as the first stage
of the amplification section but also as a switchbox that is
used to switch between
recording, stimulation, and impedance testing modes. Since
the preamplifier is
isolated from the main amplifier by an optical connection,
the preamplifier is
powered from two 9-V batteries that are located in the main
amplifier. Amplification
control is possible via the main amplifier. The gain of the
entire amplification
system varies from 100 to 10,000 times with a CMRR of 80 dB
at 1000 Hz.
The noise floor level of the system is 5 μV when the inputs
are shorted. The maximum
input to the amplifier is ±15 V, while the maximum linear
output is 20 Vp-p.
The amplifier has built-in high- and low-pass variable
single-pole filters (range:
1–500 Hz and 1–10 kHz, respectively).
The second component of the amplifier is a built-in
impedance test circuit.
This circuit passes a 30-nA (max.) current through the
electrode to ground
and has a range of 10 kΩ to 5 MΩ. Standard settings are
as follows: gain:
approximately 4000 times; high pass filter: 100 Hz; low pass
filter: 10 kHz.
 STIMULATION
STIMULATION
A number of stimulation techniques may also be performed
during movement disorder
surgery. Stimulation may be delivered via macro- or
microelectrodes and
may be used either to assess proximity to structures one
wishes to avoid (e.g.,
internal capsule, optic tract) or to assess the potential
clinical effects of chronic
stimulation. Many microelectrode recording systems allow the
surgical team to
switch between recording and stimulation modes. This permits
direct comparison
of recording and stimulation data; however, stimulation
leads to a more rapid
degradation of the microelectrode, so a new electrode may be
required for each
recording tract. Moreover, the volume of tissue that can be
affected with microelectrode
stimulation is so small that gross clinical changes are
rarely observed
with this technique. It is therefore preferable
to stimulate with
macroelectrodes, employing either the Radionics (Burlington,
MA) stimulator and
lesion generator prior to performing a neuroablation, or the
DBS lead itself when
performing a DBS procedure. Single- and dual-channel
“screener boxes” (Fig.8)
are commercially available for this purpose (Models 3625
[single lead] and 3628
[dual lead]; Medtronics Inc., Minneapolis, MN).
| |
 |
|
| |
FIGURE.8 The Medtronic’s screener boxes. The unit on the
left is a dual channel stimulator
and allows for testing two leads simultaneously. These
devices are used in the operating room to
test the location of the DBS electrode before final
implantation. The screener boxes can also be used
with the lead externalized while the patient is in the
hospital. This gives the movement disorder
team time to test parameters without permanently implanting
the whole system. |
|
During stimulation, a train of impulses is passed through
the region of interest
and the clinical effects are noted. The stimulus can be
delivered in either a
mono- or biphasic fashion. A monophasic stimulus varies from
the reference by
the signal amplitude and then returns to the reference. The
rate of change can
be edge, ramp, or sinusoidal in nature. A biphasic stimulus
varies from the reference
in both the positive and negative directions. Typically, the
amplitude of
the change is the same in both directions, but this is not
always the case. The
Medtronics, Inc. implantable neural stimulators generate a
biphasic pulse with
a positive component that is less intense than the negative
component.
Stimulation may also be mono- or bipolar in nature.
Monopolar stimulation
is generated at the active tip and is referenced to some
distant point. With bipolar
stimulation, the active and reference electrodes are in
close proximity so that
current flows within a tightly defined space. The concentric
ring electrode is a
commonly employed bipolar stimulation configuration where
the inner tip is
the active electrode and the outer ring is the reference
electrode. Chronically
implanted DBS leads are equipped with four contacts arranged
in series, allowing
for either mono- or bipolar stimulation employing any one or
combination
of contacts. In order to deliver a monopolar stimulus, the
active contact(s)
is (are) referenced to the pulse generator case. Bipolar
stimuli are conducted
between any combination of contacts. Table.1 demonstrates
some of the
important specifications for stimulators.
| TABLE.1 Stimulator Specifications |
| Feature |
First
type |
Second type |
| Output Polarity |
Bi-Phasic – Deviations in
both the positive and negative directions
from the reference point |
Mono-Phasic
– Single deviation from the reference point |
| Constant Measure |
Constant Current – The
current of the device is set by the user, and the
stimulator adjusts the voltage to compensate for
impedance deviations |
Constant
Voltage – The voltage of the device is set by the
user, and the stimulator adjusts
the current to compensate for the impedance deviations. |
| Pulse Width |
The width of each pulse |
| Frequency |
The number of pulses per second |
| Train Length |
The time that the stimulator
presents a set of pulses |
| Amplitude |
The strength of the stimulus |
| Wave Shape |
The type of waveform. Most
stimulators used for these
procedures generate square
pulses. |
 TECHNIQUE FOR MOVEMENT
DISORDER SURGERY
TECHNIQUE FOR MOVEMENT
DISORDER SURGERY
 GENERAL STEREOTACTIC TECHNIQUE
GENERAL STEREOTACTIC TECHNIQUE
The stereotactic headframe is applied on the morning of
surgery with local
anesthetic (Fig.9). Care is taken to center the head
within the frame and to
align the base ring of the frame with the orbitomeatal line,
which approximates
the orientation of the AC-PC line. In this way, axial images
obtained perpendicular
to the axis of the frame will run parallel to the AC-PC
plane. The patient is transferred to radiology, where a
stereotactic MRI is performed.
| |
 |
|
| |
FIGURE.9 The stereotactic frame with the MRI localizer
box. The plastic box is used to add
coordinate points the surgeon can use to locate objects in
the frame’s three-dimensional space. |
|
Axial fast spin-echo inversion recovery MRI
is employed to localize the
commissures and
determine their stereotactic coordinates. We then derive the
coordinates of the
midcommissural point (MCP) by averaging the coordinates of
the commissures
and calculate the coordinates of our surgical target based
on its relationship
to the commissures and/or the MCP. The calculations employed
for the
most commonly targeted sites are given in Table.2.
|
TABLE.2 Initial Target Coordinates |
| Target |
Medial lateral
coordinate |
Anterior-posterior
coordinate |
Ventral-dorsal coordinate |
| GPi |
20–23 mm from midline |
2–3 mm anterior to MCP |
6 mm
ventral to AC-PC |
| VIM |
13–15 mm from midline |
5–6 mm anterior to PC |
0 mm from
AC-PC |
| STN |
12 mm from midline |
2 mm posterior to MCP |
6 mm ventral to
AC-PC |
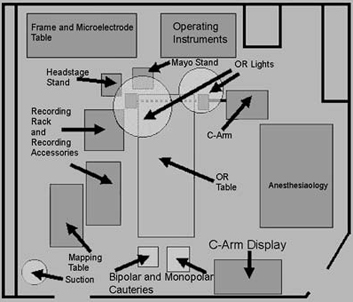
The patient is returned to the operating room (Fig.10
shows the room
layout that we employ at our center) and is positioned
supine on the operating
table, which is configured as a reclining chair for the
patient’s comfort. The target
coordinates are set on the frame, bringing the presumptive
target to the center
of the operating arc. The operation is performed through a
14-mm burr hole
that is positioned approximately 1 cm anterior to the
coronal suture and 2–3 cm
lateral of the midline. The dura mater is opened and
microelectrode recording
is begun.
| |
 |
|
| |
FIGURE.10 Layout of our operating room. This particular
setup has been found to minimize
noise. |
|
The microdrive adapter and the X-Y adjustment stage are
mounted onto the
operating arc. The microelectrode is back-loaded into the
microdrive and
zeroed to the guide tube. The electrode is withdrawn into
the cannula (∼5 mm)
for safe insertion. An insertion cannula is advanced through
the frontal lobe to
a point that is 20 mm anterosuperior to the presumptive
target. The guide tube
containing the recording electrode is inserted into the
insertion cannula and the
microdrive apparatus is mounted to the X-Y adjustment stage.
At this point the
guide tube, to the end of which the electrode tip position
is zeroed, is flush with
the end of the insertion cannula. Thus recording begins 20
mm anterosuperior
to the presumptive target.
The electrode is driven 3.0 mm into the brain and the
impedance of the
electrode–tissue system is measured. In our experience,
impedances of 700 KΩ
to 1.2 MΩ provide the best single-unit recordings. Even with
conditioning of
the electrode and stimulation testing, these starting
impedances allow for sufficient
current passage without degradation of the recording
electrode surface.
If there is a large impedance drop following electrode
conditioning, the electrode
is deemed unacceptable and is replaced. We correct any noise
problems
at this time and then proceed to data acquisition.
At the conclusion of each recording trajectory, the
collected data are
mapped onto scaled sagittal sections derived from the
Schaltenbrand-Wahren
stereotactic atlas, and a determination is made as to
tract location and
orientation employing a “best fit” model (see data
organization section). When
the data suggest that our targeting is correct, we proceed
either to test stimulation and ablation or DBS lead
insertion.
 GPi Procedures
GPi Procedures
Posteroventral pallidotomy and GPi deep brain stimulation
are reported to
improve tremor, rigidity, and LID in patients with medically
refractory, moderately
advanced PD. Though the published experience is limited,
preliminary
results suggest that GPi stimulation yields results that are
similar to pallidotomy,
with the added benefit that bilateral stimulation can be
performed more
safely than bilateral pallidotomy.
Profound improvements have also been reported in patients
with DYT1-
associated primary dystonia in whom GPi stimulation was
performed. The
authors have performed seven of these procedures, noting
dramatic improvements
in tone, posture, and overall motor function. Of course,
further study is required
before the full benefit of this surgery in primary and
secondary dystonias is known.
Successful pallidal interventions require targeting of the
sensorimotor region
of GPi, which lies posterior and ventral in the nucleus.
When recording in this
region, three key nuclear structures must be recognized: the
striatum, the GPe,
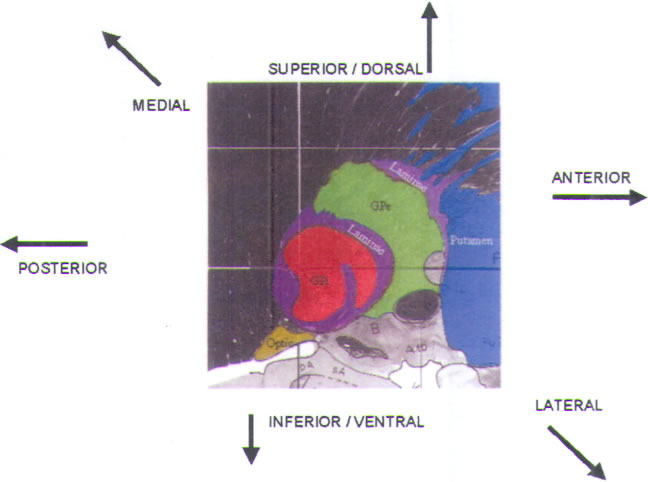
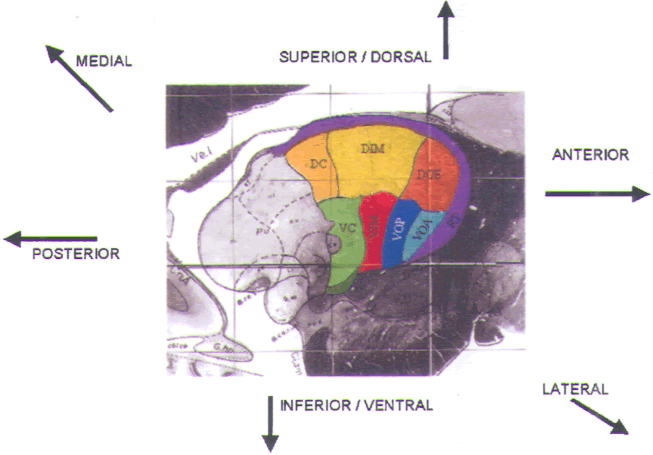
and the GPi (Fig.11) (see also color plate). Our typical
trajectory passes at a
60–70° angle above the horizontal of the AC-PC line, and at
a medial-lateral angle
of 90° (i.e., true vertical). By employing this purely
parasagittal trajectory, we can
more readily fit the operative recording data to the
parasagittal sections provided
in human stereotactic atlases.
The first cells encountered during recording are in the
corpus striatum
(caudate and putamen; colored blue in Fig.11). They
exhibit characteristic
low-amplitude action potentials, which sound like corn
popping (Fig.12A). Cellular activity in this area is extremely
scanty, and the background
is quiet. The electrode may also traverse some quiet regions
that represent
small fingerlike projections of the internal capsule into
the striatum.
| |
 |
|
| |
FIGURE.11 Sagittal slice
through the globus pallidus, taken 20.0 mm from
the midline. |
|
| |
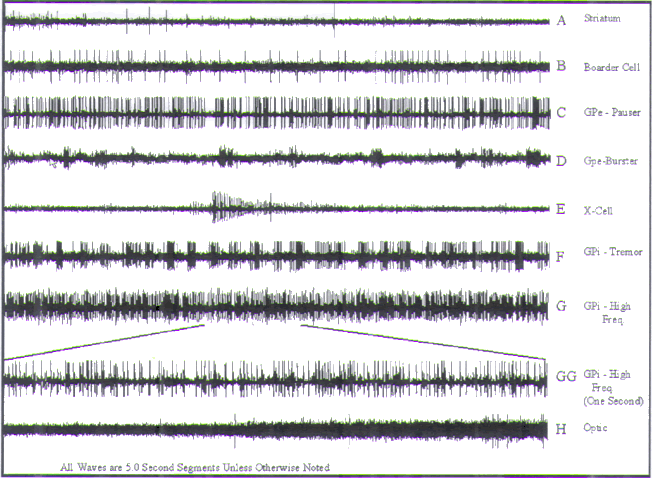 |
|
| |
FIGURE.12 Representative tracings of cellular activity
that may be encountered during a GPi
recording trajectory. Each tracing is 5 s in length, except
for trace GG, which is 1 s in length. (A)
(Sound 1) Low frequency, and sparse single spikes of the
striatum. (B) (Sound 2) Boarder cell. (C)
(Sound 3) GPe pauser cell. (D) (Sound 4) GPe burster cell.
(E) (Sound 5) The X-cell represents a
cell that is dying. (F) (Sound 6) A GPi tremor cell. (G and
GG) (Sound 7) A high-frequency cell
from GPi. (H) (Sound 8) The entry of the microelectrode into
the optic tract. The point at which
the amplitude starts to increase represents the optic tract
entry. |
|
Either the detection of a border cell or an increase in
background activity
marks entry into the GPe, the next structure to be
encountered. Border cells
(Fig.12B) exhibit very low frequencies
(between 2 and
20 Hz) that are highly periodic and high-amplitude spikes
with moderate to
wide firing times. Though rare in this region, border cells
greatly facilitate localization
of the boundaries within the globus pallidus.
Two major cell types are found within the GPe: pausers (Fig.12C) and bursters (Fig.12D). Pauser
cells fire
arrhythmically at a frequency of 30–80 Hz. They exhibit
moderate to high
amplitude discharges, a shorter time period, and lower
amplitude than the
border cells. They are distinguishable by their
staccato-type, asynchronous
pauses. An extremely small number of pauser cells (<5%) may
demonstrate
somatotopically organized kinesthetic responses.
As their name implies, burster cells are distinguished by
short bursts of
high-frequency discharges, achieving rates as high as 500
Hz. Amplitudes
vary but are usually less than the amplitudes of the pauser
cells. It is important
to differentiate bursters from what we refer to as X-cells
(Fig.12E). X-cells exhibit high-frequency discharges
(near 500 Hz)
with a time-related (<30 s) decrease in amplitude,
representing death of the
cell.
We may encounter anywhere from 4 to 8 mm of GPe during one
recording
tract. Border cells are again encountered at the inferior
border of GPe and are more
plentiful in this region. A quiet laminar area (Fig.11)
is encountered upon exit
from the GPe, marked by a steep dropoff in background
activity.
Border cells are again encountered upon entry into the GPi,
and again, two
classes of neurons predominate within the nucleus:
tremor-related cells and
high-frequency cells. Tremor cells (Fig.12F) fire rhythmically
in direct relation to the patient’s tremor. Single-unit
recordings show a
frequency modulation pattern, while semi-microelectrode
recordings show a
frequency and amplitude modulation pattern. The firing rate
of these cells is
between 80 and 200 Hz.
High-frequency cells (Fig.12G) are
characterized by
firing rates that are similar to the tremor cells (80–100
Hz), but are much more
stable, exhibiting consistent amplitude and frequency. Many
of these cells
respond to active or passive range of motion of a specific
joint or extremity.
Guridi et al. have physiologically defined a somatotopic
organization of the
kinesthetic cells in the GPi, with the face and arm region
located ventrolaterally
and the leg dorsomedially. Taha et al. found a slightly
different arrangement,
with the leg sandwiched centrally between the arm in both
the rostral and
caudal areas. Vitek et al. have found the leg to be
medial and dorsal with
respect to the arm, and the face more ventral. The GPi
is subdivided into
external and internal segments, labeled GPie (external GPi)
and GPii (internal
GPi), respectively. Both regions exhibit similar cellular
recording patterns, but
GPie may exhibit less cellularity than GPii. Total GPi
recordings normally span
from 5 to 12 mm. A steep dropoff in background activity
denotes exit from the
GPi inferiorly.
Three important white matter structures border the GPi and
may be
encountered during recording. The ansa lenticularis (AL),
which emerges from
the base of the GPi, carries motor-related efferents from
the GPi to the ventrolateral
thalamus, merging with its sister pathway, the lenticular
fasciculus
at the H field of Forel. The AL is an electrically quiet
region, although rare cells
of relatively low amplitudes and firing frequencies can be
recorded. It has been
proposed that lesioning within the AL generates the best
results from posteroventral
pallidotomy.
The optic tract (OT) lies directly inferior to the AL (Fig.11), accounting for the high rate of visual field
complications reported in
the early modern pallidotomy literature. With
quality recordings, it is
possible to hear the microelectrode tip enter the OT, the
sound of which is
reminiscent of a waterfall. Upon hearing this background
change, one may confirm
entry into the optic tract by turning off the ambient lights
and shining a
flashlight in the patient’s eyes. This will increase the
recorded signal if the electrode
is within the OT. Finally, one may encounter the internal
capsule. Background
recordings within the capsule are similar to those of the
OT. Movement
of the mouth or contralateral hemibody will generate a
swooshing sound that is
correlated to the movement. Obviously, one wishes to avoid
the posterior capsule
when making a lesion or placing a DBS lead, since a
hemiparesis or hemiplegia
may result.
Macroelectrode stimulation is performed prior to lesioning
to ensure that the
electrode is a safe distance from the internal capsule and
the OT. We conduct
test stimulation with the Radionics 1.1-mm by 3-mm
exposed-tip stimulating
and lesioning electrode,
employing a stimulation
frequency of 60 Hz and a pulse width of 0.2 ms at 0–10 V.
Stimulation of contralateral
muscular contractions at less than 2.5 V suggests that the
lesioning
electrode is too close to the internal capsule and should be
adjusted laterally.
Induction of phosphenes at less than 2.0 V suggests that the
electrode is too close
to the OT and should be withdrawn slightly. Test stimulation
should be performed
at 2- to 3-mm intervals beginning 6–8 mm above the base of
GPi as
defined by MER. Decreasing voltage trends in the induction
of muscular contractions
and/or phosphenes should be monitored. If stimulation is
begun inferiorly,
one risks creating a tract through which current may leak,
resulting in
persistently low thresholds for the stimulation of
phosphenes that will cause the lesioning probe to be
withdrawn too far. A suboptimal lesion may result.
Employing this technique,
one of the authors (RLA) has performed more than 110
pallidotomies
without inducing visual field abnormalities or hemiparesis.
If stimulation indicates that the targeted region is a safe
distance from the
internal capsule and OT, the therapeutic lesion is placed.
Ablation begins at the
base of the GPi and progresses upward in 2-mm increments,
creating a cylindrical
lesion that encompasses the span of GPi as defined by MER. A
test lesion
is initially performed at 40°C for 40 s, after which the
patient’s visual fields and
basic motor function are checked. If there are no adverse
visual field or motor
changes, a permanent lesion is performed at 80°C for 60 s.
Ideally, lesions
should not encroach upon the GPe, because the working model
of basal ganglia
physiology suggests that GPe lesioning may worsen
parkinsonism.
Excellent pallidotomy results also have been reported
without the use of
microelectrode recording and with the performance of
ablations of varying
size ranges. To date, no correlation between lesion size and
surgical outcome
has been made.
 VIM Procedures VIM Procedures
Therapeutic neuroablation or chronic high-frequency
electrical stimulation
within the ventral intermediate nucleus of the thalamus
(VIM; Fig.13) suppresses parkinsonian and essential
tremor without adversely
affecting voluntary motor activity to a significant degree
(thalamotomy may be
associated with some loss of fine dexterity). Thalamic
interventions are extremely
gratifying to perform because of the immediacy of the
results and the well-defined
physiology of the motor and sensory thalamic nuclei.
When targeting VIM, our standard angles of approach are
60–70° relative to
the AC-PC line, and 5–10° lateral of the true vertical. Pure
parasagittal trajectories
cannot be employed as they are in globus pallidus procedures
due to the
medial location of the target and a desire to avoid the
ipsilateral lateral ventricle.
| |
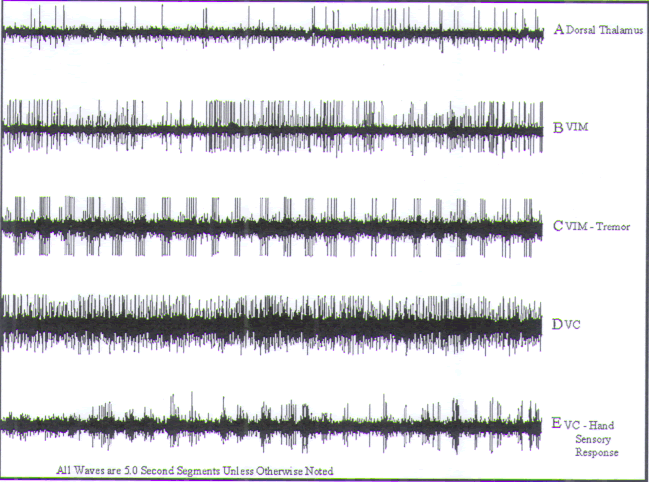
 |
|
| |
FIGURE.13 Sagittal slice through
the thalamus taken 14.5 mm from the midline. |
|
| |
 |
|
| |
FIGURE.14 Representative tracings of cellular activity
that may be encountered during a VIM
recording trajectory. Each tracing is 5 s in length. (A) Sparse dorsal thalamic cells. (B) Nontremor VIM cell. (C)
VIM tremor cell. (D) Nonsensory VC
cell. (E) Finger VC sensory cell. Note the
increase in firing rate as a light bristle paint
brush is dabbed against the finger. |
|
Transit through the ventricle may increase the risk of
hemorrhage and typically
leads to more rapid loss of cerebral spinal fluid (CSF) with
resulting brain shift
and loss of targeting accuracy.
Recording begins in the dorsal thalamus, where cells
characterized by low
amplitudes and sparse firing patterns are encountered.
Bursts of activity and
small-amplitude single spikes (Fig.14A)
are typical findings
in this region. Upon exiting the dorsal thalamus, the
electrode enters the
VL nucleus, which is composed of nucleus ventralis oralis
anterior (VOA), ventralis
oralis posterior (VOP), and VIM. The dorsal third of the VL
nucleus is
sparsely populated such that cellular recordings in this
area are similar to those
of the dorsal thalamus. As the electrode passes ventrally
within the VL complex, cellular density increases and cells
with firing rates of 40–50 Hz (Fig.14B) are encountered. Kinesthetic cells with
discrete somatotopic
representation are routinely encountered. This organization
permits an
assessment of the mediolateral position of the electrode.
The homunculus of the
ventrocaudal (Vc) and VIM nuclei are virtually identical:
representation of the
contralateral face and mouth lies 9–11 mm lateral of
midline, the arm is represented
lateral to this at 13–15 mm lateral of midline, and the leg
is more lateral
still, adjacent to the internal capsule. Thus, if one
encounters a cell that responds
to passive movement of the ankle, one knows that one has
targeted too laterally
to treat an upper-extremity tremor and should adjust the
mediolateral position
accordingly.
In addition to kinesthetic neurons, one will routinely
encounter “tremor”
cells (Fig.14C) within the VIM of
tremor patients. These
cells exhibit a rhythmic firing pattern that can be
synchronized to EMG recordings
of the patient’s tremor. Lenz et al. demonstrated that
these cells are
concentrated within VIM, 2–4 mm above the AC-PC plane, a
site that is empirically
known to yield consistent tremor control.
The recording electrode may exit VIM inferiorly, passing
into the zona
incerta (ZI) with a resulting decrease in background signal,
or it will enter Vc,
the primary sensory relay nucleus of the thalamus. Entry
into Vc is marked
by a change in the background signal. Cells in this region
are densely packed,
exhibit high amplitudes, and respond to sensory phenomena
(e.g., light
touch) with a discreet somatotopic organization, which
mirrors that of VIM and may also be used to assess target
laterality (Fig.14D). A typical cell, which responds to lightly brushing the
patient’s finger, is
featured in Fig.14E. Note the increase
in firing rate
as a light bristle paintbrush is dabbed against the finger.
The bars represent
the times that the brush is being dabbed against the finger.
If Vc is encountered
early in the recording trajectory, the electrode may be
targeted posteriorly
and should be adjusted anteriorly. The nucleus
ventrocaudalis parvocellularis
(VCpc) rests inferiorly to Vc. Recordings within this
nucleus are
similar to those of Vc; however, stimulation in this
location may yield painful
or temperature-related sensations. Single-unit recordings in
this area will
respond to both painful and temperature-related stimuli
applied within the
cell’s receptive field.
Stimulation within the thalamus for the purposes of
localizing therapeutic
lesions may be performed with constant-voltage or
constant-current
devices, and with micro- or macroelectrodes. When
stimulating with constant
current, we employ 60 μs and 1 ms pulse widths at a
frequency of 180 Hz.
Regardless of technique, the reference is a cautery ground
pad that is placed
on the back of the thigh ipsilateral to the side of the
stimulation. We consider
a motor stimulation threshold of 1 mA or 3 V safe for
placing a thalamotomy
lesion.
When performing VIM DBS, we use the lead itself to perform
test stimulation.
In such cases bipolar stimulation is performed so that a
reference pad is
unnecessary. In our experience, a properly positioned DBS
lead results in
tremor arrest at <3 V (pulse width: 60 μs; frequency: 180
Hz). Transient paresthesias
are common with a properly positioned electrode; however,
persistent
paresthesias, which are induced at low voltages, indicate
that the electrode is positioned
posteriorly, near or within Vc. Failure to suppress tremor
or induce paresthesias,
even at 5 V, suggests that the electrode is positioned
anteriorly within
VOA. Muscular contractions (typically of the contralateral
face and/or hand) suggest
that the lead is positioned too laterally and stimulation is
affecting the internal
capsule. Microelectrode stimulation
may not suppress
tremor at sites where macroelectrode stimulation is
effective.
 STN Procedures
STN Procedures
Bilateral STN DBS appears to be the most effective treatment
for PD since levodopa,
which was introduced more than a generation ago. Subthalamic
DBS
improves all of the cardinal features of PD, dampens the
severity of “on–off”
fluctuations, alleviates freezing spells, and dramatically
reduces medication
requirements.
The STN is approached at an angle of 70° relative to the
AC-PC line and
10–15° lateral of the true vertical. Microelectrode
recording begins in the anterior
thalamus and passes sequentially through the ZI, Forel’s
field H2, the STN,
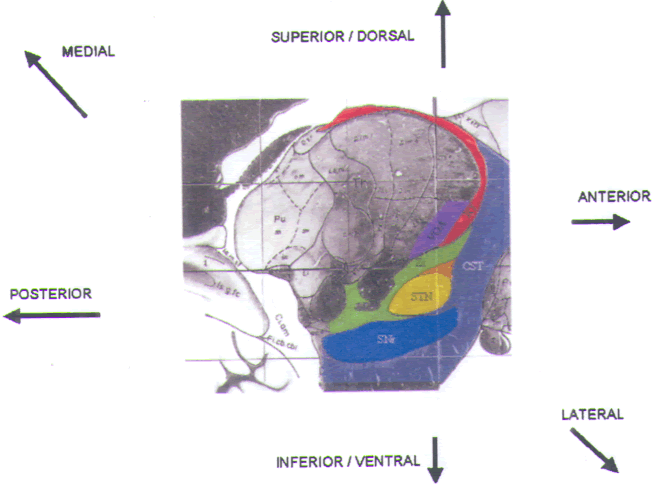
and the substantia nigra pars reticulata (SNr) (Fig.15).
In the thalamus, one encounters cells that fire with low
amplitude and frequency.
Two patterns of activity may be identified: (1) bursts of
activity(Fig. 16A) and; (2) irregular,
low-frequency (1–30 Hz)
activity (Fig.16B). The density of cellular activity varies
in this region. For example, it is
observed that VOA is more cellular than the reticular
thalamus.
The border between the thalamus and ZI (Fig.16C)
may be very distinct, but not in all cases. Developmentally,
the ZI is a continuation
of the reticular nucleus of the thalamus, and the transition
from one to the
other may not be clear. The ZI can be differentiated
electrophysiologically from
the thalamus in two ways. First, cellular activity is more
muffled or “muddy” in
the ZI. By this we mean that the cellular firing rates slow
and become a little more
asynchronous, and the amplitudes decrease in intensity.
These changes are
subtle and can be missed by inexperienced observers. The
second indication
of transition from thalamus to ZI is a change in the
background recordings.
| |
 |
|
| |
FIGURE.15 Sagittal slice
through the STN taken 12.0 mm from the midline. |
|
Whereas the background of the thalamus proper is somewhat
active, the ZI
background is much quieter. Typically, the recording
electrode will exit the
thalamus 6–10 mm anterosuperior to our presumptive target
and will pass
through 2.5–4.0 mm of ZI before entering H2. If more than 4
mm of relative
“quiet” is encountered, a trajectory that is anterior or
posterior to the STN
should be suspected.
A decrease in background activity demarcates entry into
Forel’s field H2,
which lies immediately superior to the STN, 10–12 mm lateral
of midline.
Sparse cellular activity is detected over a span of 1–2 mm.
Background activity
increases as the recording electrode enters STN.
Additionally, dense cellular
activity is now encountered. Two patterns of cellular
activity are observed
within STN: (1) tremor activity (Fig. 16.16D, CD-STN sound
4) similar to that encountered in VIM or GPi; and (2)
single-cell activity (Fig.16E) with frequencies that vary from ∼25 Hz to 45 Hz.
Cells in the dorsal
segments of the STN exhibit slower firing rates than those
of the ventral STN. Kinesthetic related activity is often observed, but a clear somatotopy is not evident.
Upon exiting the STN, the microelectrode may pass through a
thin quiet
zone or will pass directly into the SNr. Entry into the SNr
is demarcated by significant
increases both in background neural activity and in cellular
firing rates
(Fig.16F), which are usually greater
than 60 Hz. Up to
7 mm of SNr may be encountered, depending on the
anteroposterior position
of the trajectory.
| |
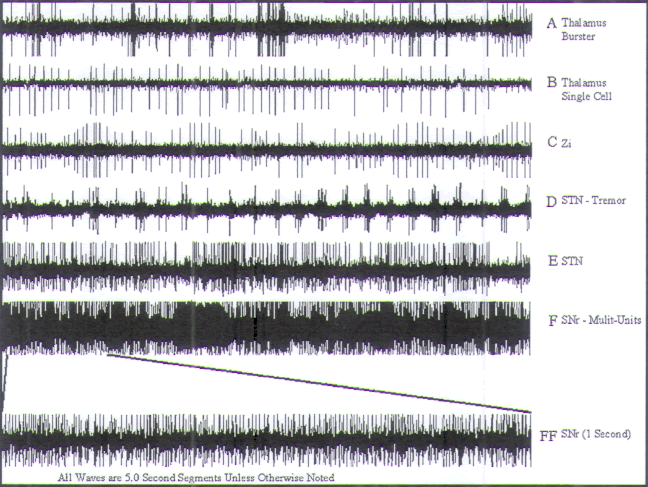 |
|
| |
FIGURE.16 Representative tracings of cellular activity
that may be encountered during a STN
recording trajectory. Each tracing is 5 s in length, except
for trace FF, which is 1 s in length. (A) Thalamic burster cell and single cell. (B)
Thalamic single cell. (C) ZI cellular activity. (D) STN tremor cell.
(E) Nontremor STN cell from
the ventral half of the STN nucleus. (F) (Sound 19) SNr cell |
|
Required 4–6 mm of STN, preferably with evidence of
kinesthetic activity,
for implantation of the DBS lead. This large a span allows
for two of the four
electrode contacts to be placed within the nucleus, leaving
the other two above
the nucleus in the ZI and H2. Additionally, this large a
span of STN recording
ensures that the electrodes are implanted solidly within the
nucleus and not
near a border.
The primary goal of test stimulation at the STN is to check
for stimulation-induced
adverse events (AEs) because, aside from tremor arrest and
some
modest reductions in rigidity, positive STN stimulation
effects may not be
observed for hours or days. Test stimulation is performed in
bipolar configuration
with the implanted DBS lead and Medtronic’s single lead
screener (model 3625,
Medtronic, Minneapolis, MN). Parameters are: 60 μs, 180 Hz,
0–4 V. We do not
stimulate higher than 4.0 V for fear of inducing hemiballism.
Moreover, we
have yet to employ amplitudes greater than 4 V to achieve
clinical benefit at this
target. Transient paresthesias are frequently encountered
with the onset of stimulation.
Persistent paresthesias indicate stimulation of the medial
lemniscal
pathway, which lies posterolateral to the nucleus.
Stimulation-induced contractions
of the contralateral hemibody and/or face indicate
anterolateral misplacement
of the lead. Finally, abnormal eye movements may be
encountered
if the lead is positioned too medially or deep to the
nucleus. The first test stimulation
is performed using contacts 0−, 1+ up to a voltage of 4.0 V.
If no significant
adverse effects are encountered with this focal test, we
proceed to test
stimulation employing all four contacts (i.e., 0−, 1−, 2+,
3+ up to a voltage of
4.0 V). This test covers the full contact space of the
electrodes and focuses on
identifying stimulation-induced adverse events in the
ventral aspect of the stimulation
field. This is the area where most AEs have occurred in our
experience.
The final stimulation is performed using contacts 0+, 1+,
2−, 3− up to a voltage
of 4.0 V. This examines the dorsal aspect of the stimulation
field.
 Data Organization
Data Organization
The data from each microrecording tract are plotted on
scaled graph paper
(1.0 cm: 1.0 mm). The borders of each encountered
structure are
marked, and the span of each region is represented by a
different color for
easy differentiation. In order to accurately account for our
angle of approach,
a line that is parallel to the intercommissural line is also
drawn. The plotted
tract is then traced onto a transparent plastic sheet. The
transparency is
placed on scaled maps (10:1) derived from the
Schaltenbrand-Wahren human stereotactic atlas
in order to
determine to which map the trajectory best fits. The
accuracy of the fit is
dependent upon the number of trajectories, the number of
structures encountered
along each trajectory, and finally upon how well the
patient’s anatomy
fits the atlas, which is derived from a single human
specimen. It can be difficult
to find one place to which a single tract fits best,
especially when performing
pallidal or thalamic interventions. When mapping the STN,
the many
structures encountered along a single trajectory make
fitting it to the atlas a
little more straightforward. If there is any question about
the proper fit of the
data, we perform another recording tract. Knowing the
spatial relationship
between each tract, we can better fit all of the data to the
atlas with each subsequent
trajectory.
| |
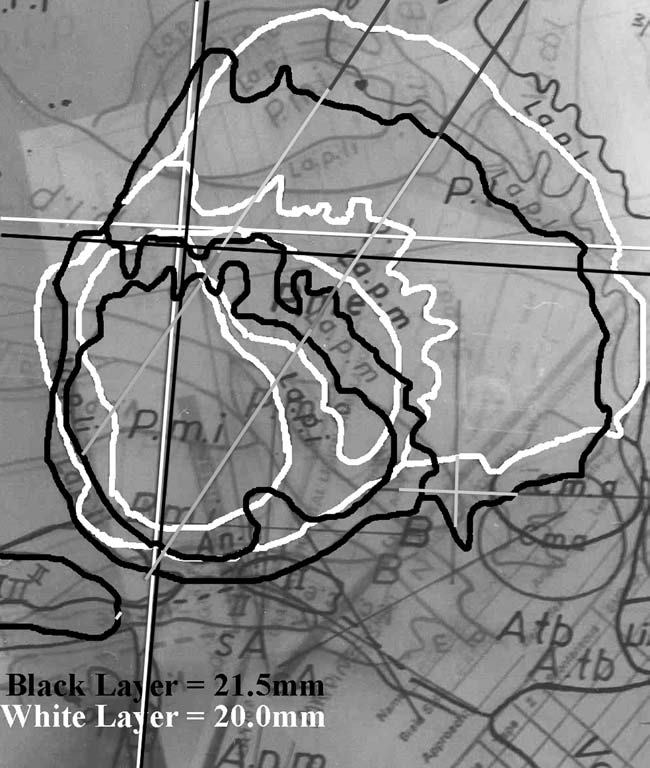 |
|
| |
FIGURE.17 Once the recording data are transferred to 1:10
scaled graph paper, the trajectories
are transferred to a transparency. The angle of the
trajectory relative to the AC-PC line is added
to the transparency, and the trajectory is then fitted to
scaled atlas sections. This figure shows two
trajectories during a GPi lesion surgery. The green lines
represent the GPe part of the trajectory,
and the red lines represent the GPi part of the trajectory.
By overlaying two atlas maps, a three dimensional picture of
the trajectories can be formed. |
|
 CONCLUSION
CONCLUSION
The fine details of these procedures vary from center to
center, but the
neurophysiological techniques used by each center can be
divided into the
following categories: (1) microrecording; (2) semi-microrecording;
(3) stimulation;
and (4) evoked response testing. In the over 1,500
trajectories performed
by Shils J. L. et al., they feel that the information gathered with microrecording is
of great benefit when performing these surgeries.
Microrecording has been
shown to be as safe as other stereotactic procedures
when done properly.
With these surgeries they tried to modify the physiology
of a target
structure; therefore, microrecording gives specific
physiologic data to help
determine the optimal placement. In most cases (43–88%,
depending on the
study), this physiological target corresponds
to the anatomic
target, but in the 12–67% of cases that is not the case. At
present there is no
way of knowing which of these patients will fall into either
category before the
surgery.
The neurophysiologic techniques used in the operating room
require trained
and skilled personnel, not only to acquire but also to
interpret the data. If everything
goes perfectly, the data are relatively easy to interpret,
but when the signals
are not textbook cases, this interpretation needs to be done
by very
experienced personnel. Up until the mid-1990s, centers had
to put their own
microelectrode recording systems together and build their
own microelectrodes,
since there were no commercially available systems. At the
present time
there now exist about 10 companies (internationally) that
produce microrecording
systems, and the first FDA-approved microelectrodes were
placed on
the market in 2000. The key points to get the best signals
at are the microelectrode,
preamplifier, and amplifier. The main feature of reliable
microelectrode
systems for neurophysiological targeting of deep brain
structure is the quality
of the recorded signal. This is more important than any of
the fashionable features
that many manufactures offer. No software-based
interpretation scheme is
going to replace the skilled human interpreter when the
recordings are difficult.
As already stated, the operating room is very harsh
electrically. The more we
learn about the areas of interest, the faster and smoother
each of these procedures
will go. |


 INTRODUCTION
INTRODUCTION
















 What’s Up
What’s Up